Each year, the Grand Ouest Committee of the League Against Cancer actively participates in research by financing a certain number of scientific projects. At the start of 2024, he donated €84,000 to CBM researchers.
3 projects, at the cutting edge of cancer research, have been funded.
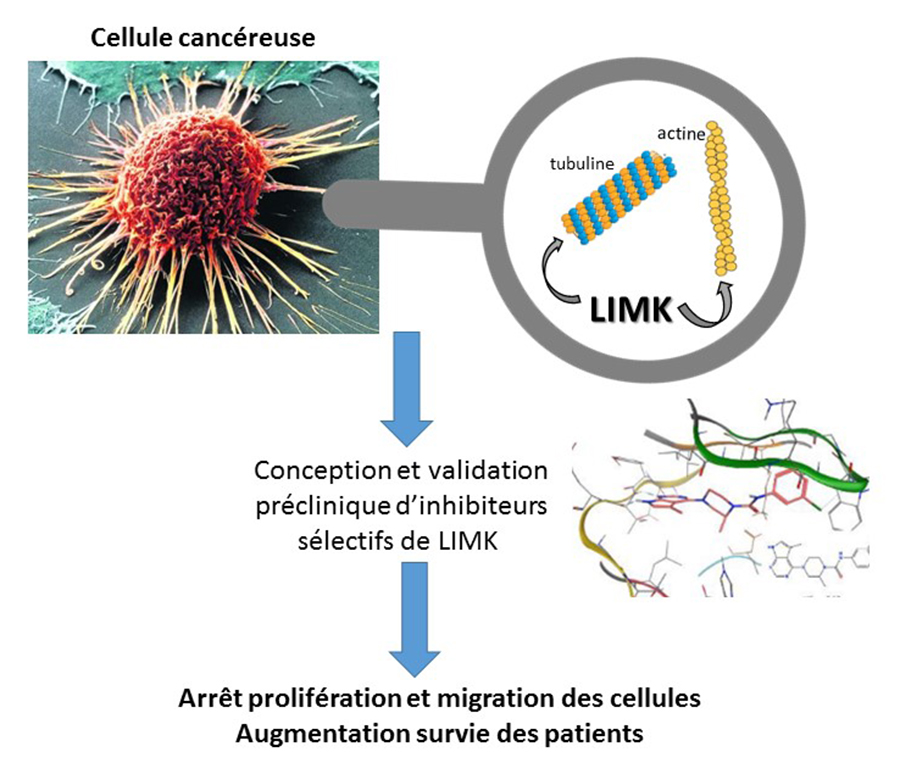
"New therapeutic approach in oncology: development of PROTACs targeting LIMK1 and LIMK2 kinases"
Leader: Béatrice Vallée co-leader of the “Cell signaling and neurofibromatosis” team
Partner: Karen Plé, ICOA, UMR7311, CNRS/University of Orléans
The protein kinases, LIMK1 and LIMK2, are involved in cell skeletal remo Their active role in the development of cancers has been shown both in the formation of tumors and in their dissemination and the growth of metastases. Targeting LIMK1 and LIMK2 to develop new anti-cancer therapies is therefore a very relevant strategy.Unfortunately, traditional small chemical molecules inhibiting the activity of LIMK1 and LIMK2 have not passed clinical trials.In our project, we therefore decided to develop a new class of molecules, PROTACs, which allow the destruction of their target directly in the cell. In our case, we target the destruction of LIMK1 and LIMK2 to annihilate their oncological activity. This PROTAC strategy is very innovative and growing. Building on very promising results that have been obtained on other therapeutic targets, we hope to demonstrate the proof of concept of this new approach targeting LIMK1 and LIMK2, and thus open the way to new therapeutic molecules.
This project received the support of the League against Cancer, the Committees of Loiret, Loir-et-Cher and Morbihan, for the sum of €32,000.
“Innovative prostate cancer diagnosis for personalized medicine approaches: intelligent multiplex mapping of SKCa channels using near-infrared emitting lanthanide-based metallacrowns”
Leader: Svetlana ELISEEVA, “Luminescent lanthanide compounds, spectroscopy and optical bioimaging” team
Each patient's tumors are different because each individual is different. It is therefore crucial to take this diversity of tumors into account to develop personalized approaches. Our research work aims to characterize cancerous tumors with the aim of providing more effective treatment to cure cancer.Several specific potassium channels play a major role in the progression of cancerous tumors and are sensitive to therapies. In this project, we are implementing an innovative near-infrared optical imaging approach which will make it possible to establish the precise identity map of these potassium channels for each patient in order to provide personalized treatment.
This project received the support of the League against Cancer of the Committees of Loiret, Loir-et-Cher and Sarthe, for the sum of €30,000.
“Cancer immunotherapy: evaluation of synthetic versus in-cell expressed bispecific di-affibodies”
Leader: Josef Hamacek, head of the “Molecular assemblies and complex systems” team
Partners: Federico Perche, Vincent Aucagne (CBM), Florence Velge-Roussel (NMNS Tours)
Several receptors on the surface of cancer cells may represent potential targets for antibodies with broad neutralization spectrum. The principle of bispecific synergistic antibodies is to bind via a binding site on the surface of the tumor cell and with the other site to a receptor on the surface of the immune effector cell (NK, T lymphocytes).In this context, antibodies can be replaced by affibodies (AfBs) presenting affinities and selectivities for their targets comparable to antibodies, but having smaller size.This project aims to develop bispecific di-AfBs as innovative agents for the diagnosis and immunotherapy of cancer.These complex molecules are made up of two AfBs linked by linkers and can thus target two epitopes. AfBs will bind specifically to the corresponding receptors overexpressed on the surface of cancer cells to block signaling pathways, and to promote the recruitment of effector cells.This concept offers new therapeutic perspectives and makes it possible to optimize interactions with the cell and provoke the immune reaction.
This project received support from the League Against Cancer of the Committees of Loiret, Sarthe and Côtes d’Armor, for the sum of €22,000.