Lylia Azoug, PhDstudent in the “Synthetic protein and bioorthogonal chemistry” team, received the poster prize during the “Chemical Biology Symposium 2024” conference organized by the Société Chimique de France.
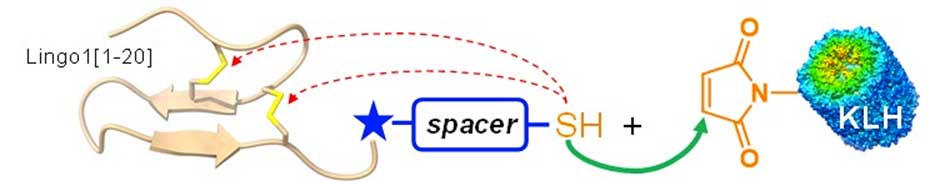
Poster abstract :
Generation of specific antibodies against peptides requires their covalent conjugation to large protein carriers, such as keyhole limpet hemocyanin (KLH), to override their inherently weak immunogenicity. For this, most approaches exploit the highly-efficient thiol-maleimide ligation, which enables for the attachment of multiple copies of peptides displaying a single cysteine thiol group, onto a variety of commercial maleimide-functionalized carriers. Concerning disulfide-containing peptides and proteins, introduction of a spare thiol moiety permits a follow-up thiol-maleimide reaction; nevertheless, disulfide bonds are highly susceptible to undergo intramolecular thiophilic attack by the thiolate required for the reaction, with consequent impairing of their native bridging framework. This “disulfide scrambling” would alter the peptide 3D structure, impairing specificity of elicited antibodies for the native target. In recent reports, thiol-functionalized spacers were introduced on disulfide-containing peptides to generate protein conjugates for immunization purposes, 1,2 nevertheless the occurrence of disulfide scrambling was not examined, therefore we sought to systematically evaluate the influence of different thiol-functionalized spacers. Using the N-terminal domain of the Lingo1 protein as a model peptide (20 amino acids, 2 disulfide bridges), we probed a small battery of thiol-containing spacers with variable length and rigidity. Interestingly, while flexible linkers, comparable with previously described ones,1,2 showed considerable disulfide scrambling upon 2 h under thiol-maleimide reaction conditions (pH 6.6), a short and rigid oligoproline-based linker3 abolished the disruption of the disulfide framework. The reported strategy provides a valuable resource for the bioconjugation of disulfide-containing peptides via standard thiol-maleimide chemistry while maintaining the structural integrity of the disulfide framework, thereby providing homogeneous tools for research and biomedical applications.
- Katayama, H.; Mita, M. A sulfanyl-PEG derivative of relaxin-like peptide utilizable for the conjugation with KLH and the antibody production. Bioorganic & ; Med. Chem. 2016, 24 (16), 3596–3602. DOI : 10.1016/j.bmc.2016.05.068
- Katayama, H.; Mizuno, R.; Mita, M. A novel approach for preparing disulfide-rich peptide-KLH conjugate applicable to the antibody production. Biosci. Biotechnol. Biochem. 2019, 83 (10), 1791–1799. DOI : 10.1080/09168451.2019.1618696
- Wilhelm, P.; Lewandowski, B.; Trapp, N.; Wennemers, H. A Crystal Structure of an Oligoproline PPII-Helix, at Last.J. Am. Chem. Soc. 2014, 136 (45), 15829–15832. DOI : 10.1021/ja507405j