FAM118B and FAM118A : new sirtuins of bacterial origin, filament, in the heart of human cells
Published on Jan. 28, 2026FAM118B, initially discovered in Cajal bodies — nuclear structures involved in the distribution and organisation of nuclear factors — is characterised in this study, alongside its paralogue FAM118A, as a new human sirtuin.
Sirtuins are a family of enzymes that consume NAD⁺ and regulate numerous cellular processes. Present in all domains of life, there are seven sirtuins in humans. This study identifies FAM118A and FAM118B as two additional human members, similar to bacterial sirtuins in structure and properties — making these proteins true ‘molecular fossils’ that have diverged little from their bacterial ancestor.
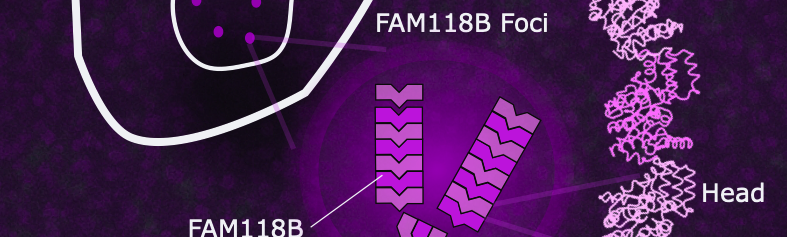
FAM118B and FAM118A individually form homogeneous filaments, a characteristic shared with bacterial sirtuins involved in defence against phages, even though their polymerisation mechanisms differ. Although FAM118B and FAM118A exhibit low enzymatic activity when isolated, their synergistic association triggers high NAD⁺ consumption, revealing a cooperative mechanism based on the formation of heterogeneous filaments in cells.
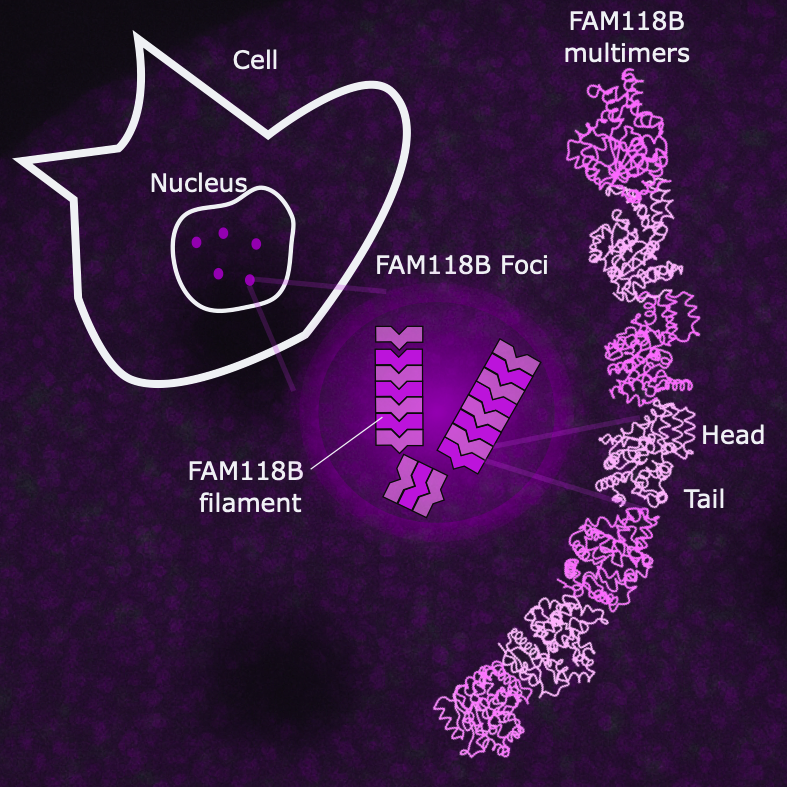
FAM118B is located in the nucleus. Its oligomerisation, often transient, leads to the formation of nuclear foci. The structure obtained by cryo-electron microscopy reveals an end-to-end organisation of protomers that stabilises the filament.
This study draws on a range of complementary techniques: cryo-electron microscopy, biochemistry, microscopy and various forms of in cellulo fluorescence spectroscopy, and functional analyses in human cells. It is the result of an international collaborative effort bringing together teams from Orléans, Oxford, Rennes, Paris, and Croatia, with Domagoj Baretić and Sophia Missoury as co-first authors.
By combining in vitro and in cellulo approaches, this work highlights a new function of human sirtuins, at the interface between nuclear structure and metabolism, and opens up new perspectives for better understanding their role in health, immunity, and cell regulation.