Theme 2.1: Immunotargeted injectable therapies
Objectives
- Innovate therapeutic strategies (new antibody formats, co-treatment, theranostics)
- Improve immunotargeting (selectivity)
- Increase anti-tumoral effect
- Decrease chimioresistance
- Reduce treatment side effects
- Generate relevant in vitro models (2D, 3D spheroïd models)
- Use innovative evaluation methods (imaging)
Summary
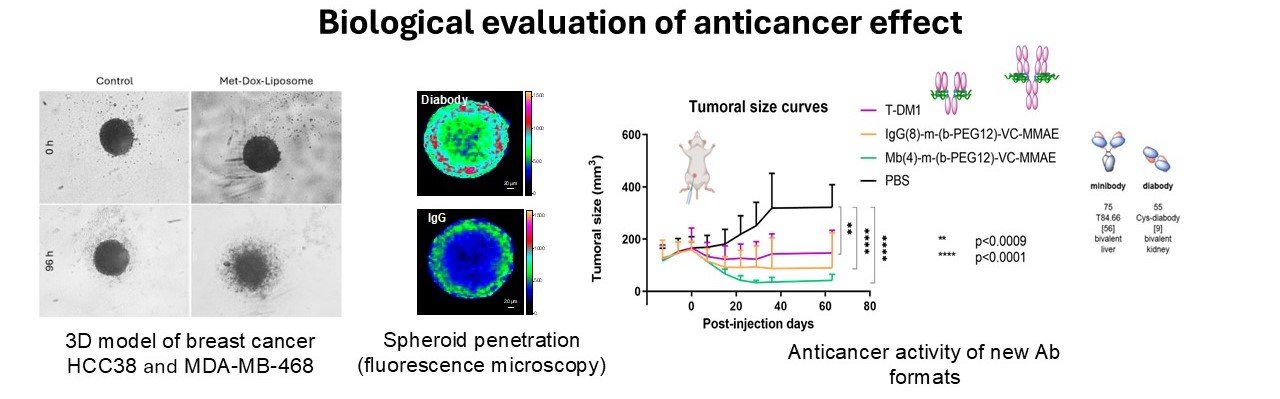
This theme focuses on the biological evaluation of innovative strategies for anticancer therapies. Among these therapies, we can find various nano-objects encapsulating active pharmaceutical ingredient (API) that have been functionalized with antibody fragments (scFv) targeting different proteins (EGFR, HER2, TROP-2), and antibody or fragment drug conjugate (ADC or FDC). The objectives of this theme is to study the trafficking of such nanotherapies on different models (breast and lung models) and to evaluate the antitumoral effect of these nanobiotherapies.
Among the biological evaluations, we can perform :
- 2D cell culture evaluation : affinity for the targeted proteins (ELISA, BLI), cell internalization (cytometry, fluorescence imaging and confocal spectral imaging), cell cytotoxicity (viability or cytotoxicity tests, apoptosis tests), bystander tests on co-cultures.
- 3D cell culture on spheroids (homo or heterotypic): cell model characterization (cytometry, immunofluorescence, immunostaining), penetration study (confocal spectral imaging, confocal imaging), 3D viability tests.
- In vivo evaluation on mice : tumoral reduction study on mice xenografted models after the injection of the nanobiotherapies (by IV or IM route) and biodistribution study by imaging (IRM, fluorescence, SPECT-CT).
Keywords
- Targeted Nanomedicine
- Anticancer Immunoconjugates (ADCs and FDCs)
- Antibody Fragments (scFv, VHH)
- Penetrating Peptides (CPPs)
- Tumor Antigens (HER2, HER3, TROP 2, EGFR)
- Nucleic Acids (siRNA, miRNA, DNA)
- Anticancer Agents (DOX, PTX, TAK981…)