PEPTIDES ANTIMICROBIENS (RICHES EN PONTS DISULFURE)
Les peptides antimicrobiens (PAMs), molécules clé de l’immunité innée de tous les organismes vivants, représentent aujourd’hui une des alternatives prometteuses pour lutter contre l’antibiorésistance, problème majeur de santé publique.
Nous travaillons à la compréhension fine des structures (diversités structurales, relations structures-activités, évolution des structures via la phylogénie) et la compréhension fine des mécanismes d’action de certains PAMs originaux prometteurs, pour participer à la conception des nouveaux composés antimicrobiens de demain.
Les 4 exemples ci-dessous (double défensines d’oiseaux, défensines du manchot Royal, défensines d’huitre, défensines de papillons) illustrent la complémentarité des techniques que nous associons à la RMN - phylogénie, imagerie, thermophorèse à micro-échelle (MST) et calorimétrie (ITC) – pour disséquer ces mécanismes d’action à l’échelle moléculaire et atomique.
Les doubles défensines d’oiseaux
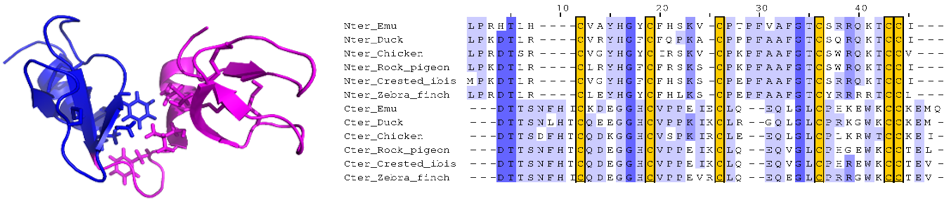
Nous avons étudié la structure 3D de la première double défensine aviaire, Gga-AvBD1, du poulet Gallus gallus, défini un nouveau repliement 3D non-répertorié dans la Protein Data Bank. Nous avons étudié plusieurs activités biologiques, en particulier antibactériennes, antiparasitaires et antivirales, et évalué la contribution de chacun des domaines à ses activités (gauche, www.pnas.org/cgi/doi/10.1073/pnas.1912941117). Enfin, nous avons montré que le gène de cette double-defensine, présent dans tous les génomes d’oiseaux actuellement répertoriés (plus de 300), ne provient pas d’une duplication-fusion (droite, https://doi.org/10.3390/biology11050690).
Les défensines ancestrales d’huitres
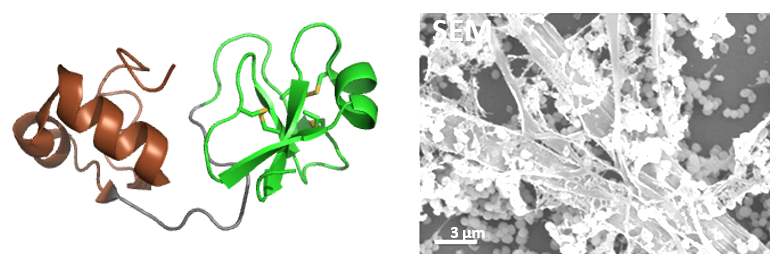
Nous avons résolu la structure 3D de deux big-défensines de l’huître Crassostrea gigas, actives en milieu salé (400 mM NaCl) contre un large spectre de bactéries (exemple Cg-BigDef1, à gauche, https://doi.org/10.1128/mBio.01821-19). Ces PAMs à deux domaines comportent un domaine β-défensine-like (en vert) riche en pont disulfure et un domaine N-terminal hydrophobe, très conservé, structuré en hélice-tour-hélice (en brun). Ce dernier est indispensable pour l’activité antimicrobienne en milieu salé, et qu’il gouverne l’auto-association de la molécule entière en formant des « nanonets » pour piéger les bactéries (à droite). Nous avons aussi montré une synergie d’action entre les domaines, et une complémentarité remarquable de leur spectre antimicrobien https://doi.org/10.1128/mBio.01821-19, https://doi.org/10.3390/md20120745). Enfin, sur l’exemple de Staphylococcus aureus nous avons montré le rôle clé des acides teichoïques dans le mécanisme (https://doi.org/10.1021/acsinfecdis.5c00646).
Les défensines du Manchot Royal
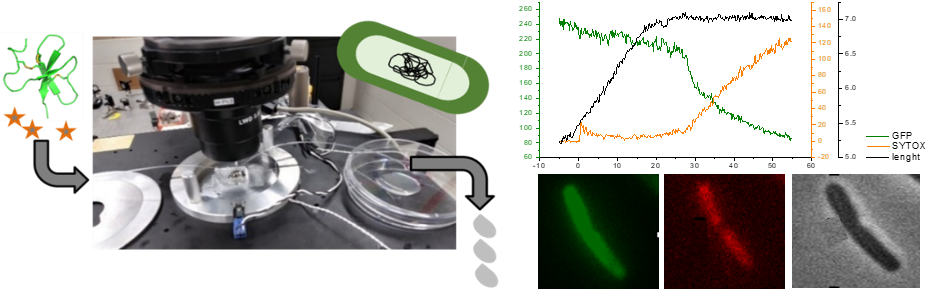
Une première étape essentielle vers l'ingénierie rationnelle de peptides antibactériens hautement efficaces à usage clinique consiste à comprendre en détail le fonctionnement des PAMs optimisés par la nature. Les données concernant les PAMs non lytiques (cad qui ne lysent pas la membrane bactérienne pour détruire la bactérie), tels que les β-défensines, sont rares et restent donc mal comprises. Nous étudions le mécanisme d’action des défensines, sur l’exemple de la défensine AvBD103b du Manchot royal, en réalisant des expériences de microscopie de fluorescence sur bactéries E. coli vivantes qui nous permettent de suivre en temps réel la moindre perméabilisation de leurs membranes interne et externe (https://doi.org/10.3390/ijms23042057).
Les défensines de papillons
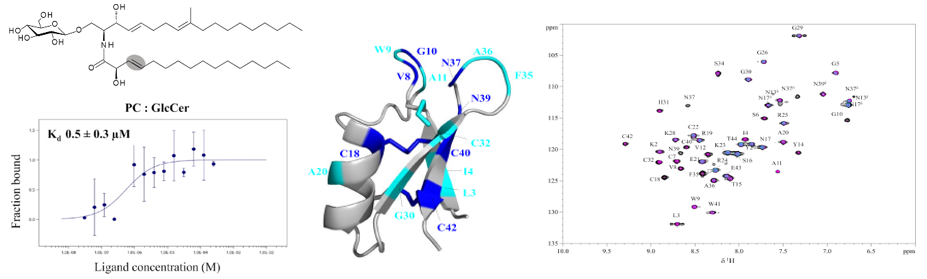
La défensine ETD151 a été optimisée à partir de défensines de papillons. Nous avons mis en évidence que la première étape de son mécanisme multifacette nécessitait la présence de glucosylcéramides (GlcCers), lipides de la membrane fongique déterminants pour la croissance et la virulence du champignon, et donc considérés comme des cibles prometteuses. Nous avons montré qu’il existe une interaction moléculaire directe entre ETD151 et le GlcCer fongique (https://doi.org/10.1073/pnas.2415524122), et nous l’étudions par une approche multidisciplinaire, qui combine la thermophorèse à micro-échelle (MST), le titrage calorimétrique isotherme (ITC), la RMN en solution et la RMN du solide. Nous avons montré que le méthyle, spécifique des GlcCer de champignons, jouait un rôle majeur dans cette interaction (https://doi.org/10.1016/j.jbc.2025.110587).