AUTRES PEPTIDES RICHES EN PONTS DISULFURE (TOXINES)
Les toxines riches en pont disulfure ont de fortes identités structurales avec les peptides antimicrobiens décrits ci-dessus. Les toxines que nous étudions sont de diverses origines (plantes, fourmis, araignées, scorpions).
Nous travaillons à la compréhension fine des structures (diversités structurales, relations structures-activités, évolution des structures au cours de l’évolution des espèces) pour améliorer la compréhension des mécanismes d’action et participer à la conception des nouveaux composés insecticides de demain. Enfin, nous participons à plusieurs projets sur des toxines photo-activables, ce qui ouvrent de nouvelles voies thérapeutiques.
Toxines insecticides de plantes
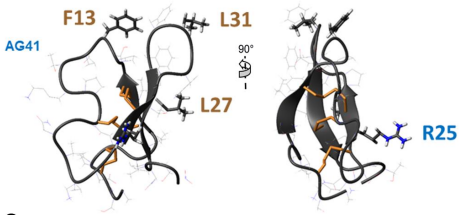
Les biopesticides sont des alternatives efficaces aux insecticides chimiques. Nous étudions le peptide AG41 provenant des racines de la luzerne qui présente une activité insecticide exceptionnelle contre plusieurs ravageurs tels que les pucerons et les charançons. La structure 3D que nous avons déterminée a permis d’établir des relations structure-fonction qui expliquent cette forte activité insecticide. Les résidus clés pour l'entomotoxicité du peptide AG41 sont F13, R25, L27 et L31 (https://doi.org/10.3390/biom13030446).
Toxines insecticides de venins de fourmis
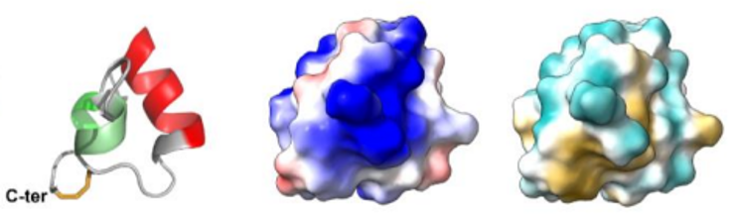
Les venins de fourmis, cocktails de toxines optimisés pour perturber les systèmes physiologiques des insectes proies, constituent un réservoir très prometteur pour la découverte de nouveaux insecticides. Parmi les peptides que nous avons étudiés, nous avons montré que le peptide U11 est l’un des plus paralysants jamais rapportés à partir de venins de fourmis contre les mouches à viande. Le peptide U11 a une structure hélicoïdale compacte unique, stabilisée par un pont disulfure (de gauche à droite la structure 3D du peptide U11, les potentiels électrostatiques à la surface, les potentiels hydrophobes à la surface) (https://doi.org/10.3390/toxins15100600)
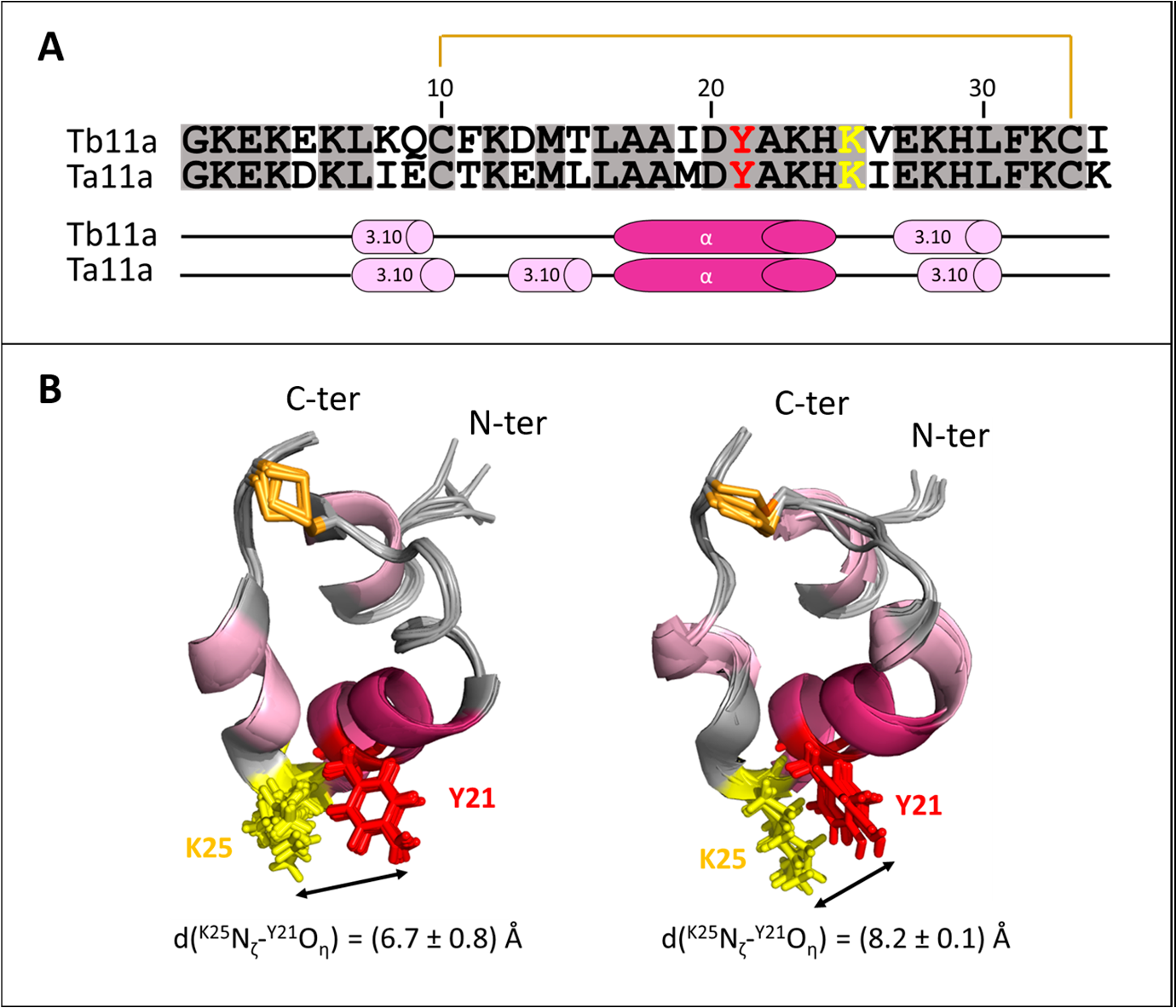
Toxines de scorpion ou d’araignée photo-activables
L’objectif de ces projets est de cibler les canaux avec un contrôle spatiotemporel très précis, ce qui ouvre de nouvelles opportunités d’interventions thérapeutiques « light-guided ».
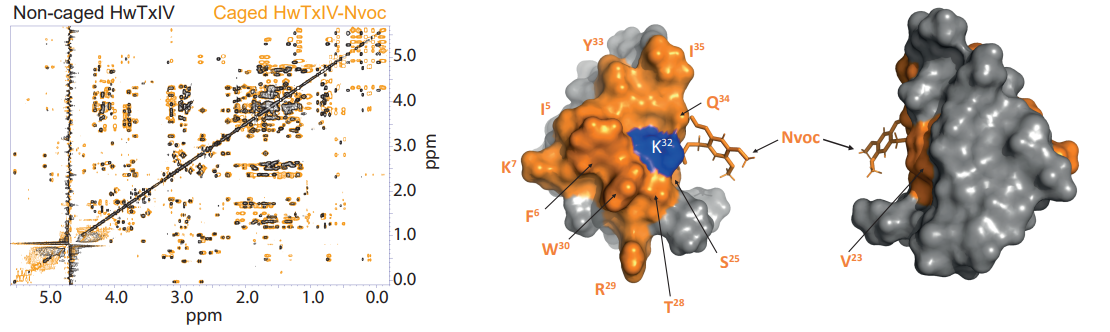
Dans un premier temps, notre rôle a été de valider le greffage du groupement Nvoc (UV-clivable) sur la toxine Huwentoxin-IV (HwTx-IV), de l’araignée Cyriopagopus schmidti, et son total clivage (après UV), sans impact structural sur la toxine. A gauche exemple de superposition de spectres RMN d’une toxine s’araignée ciblant les canaux ioniques neuronaux, libre ou liée au groupement Nvoc photo-activable. A droite, structure 3D indiquant la zone perturbée par la présence du groupement photo-activable (https://doi.org/10.1038/s41467-022-27974-w ).
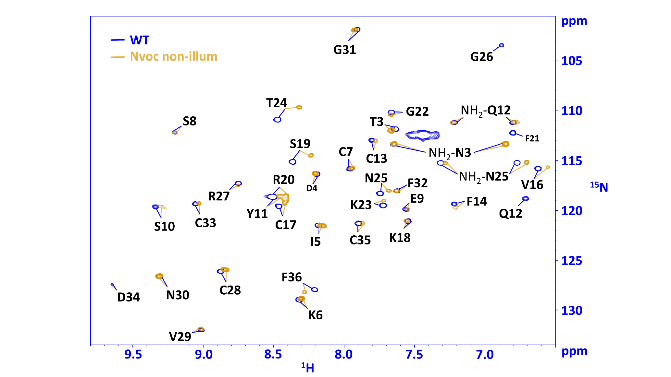
Après cette preuve de concept, nous avons réalisé l’application sur la toxine BeKm-1 du scorpion Buthus eupeus, active sur le canal ERG (https://doi.org/10.1161/CIRCRESAHA.123.322880).
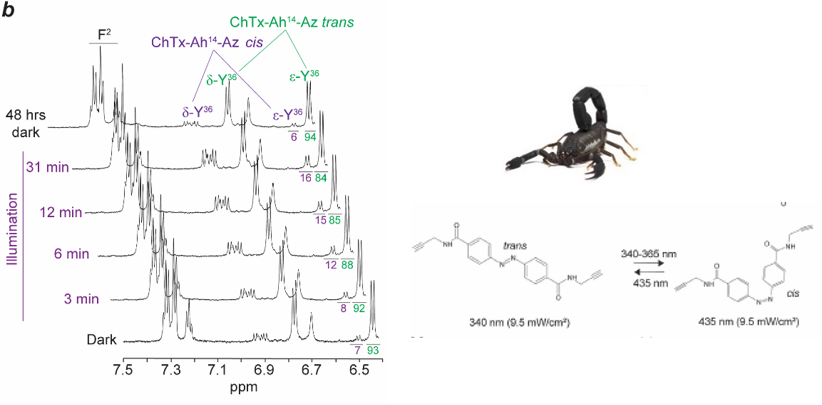
Enfin, nous avons suivi par RMN l’isomérisation réversible au niveau du groupe Azobenzène gréffé sur la Charybdotoxine ChTx, une toxine du scorpion Leiurus quinquestriatus hebraeus, active sur le canal Kv1.2. L'intégration réussie dépend du positionnement précis de l'azobenzène et du greffage chimique guidé par les connaissances SAR. Cette avancée souligne l'adaptabilité de la technologie des photoswitchs à structures peptidiques complexes, offrant ainsi de nouvelles possibilités de modulation pharmacologique (https://doi.org/10.1002/anie.202423278).