Zaïm, A., Nozary, H., Guénée, L., Besnard, C.,Lemonnier, J.F., Petoud, S. and Piguet, C.
Chemistry a European Journal (2012) 18, 7155-7168
Résumé :
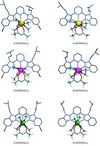
Herein, we discuss how, why,and when cascade complexation reactions produce stable, mononuclear, luminescent ternary complexes, by considering the binding of exafluoroacetylacetonate anions (hfac-) and neutral, semi-rigid, tridentate, 6-bis(benzimidazol-2-yl)pyridine ligands (Lk) to trivalent lanthanide atoms (LnIII). The solid-state structures of Ln(Lk)ACHTUNGTRENUNG(hfac)3] (Ln=La, Eu, Lu) showed that [Ln-ACHTUNGTRENUNG(hfac)3] behaved as a neutral six-coordinate lanthanide carrier with remarkable properties: 1) the strong cohesion between the trivalent cation and the didentate hfac anions prevented salt dissociation; 2) the electron-withdrawing trifluoromethyl substituents limited charge-neutralization and favored cascade complexation with Lk; 3) nine-coordination was preserved for [Ln(Lk)-ACHTUNGTRENUNG(hfac)3] for the complete lanthanide series, whilst a counterintuitive trend showed that the complexes formed with the smaller lanthanide elements were estabilized. Thermodynamic and NMR spectroscopic studies in solution confirmed that these characteristics were retained for solvated molecules, but the operation of concerted anion/ ligand transfers with the larger cations induced subtle structural variations. Combined with the strong red photoluminescence of Eu(Lk)ACHTUNGTRENUNG(hfac)3], the ternary system LnIII/hfac-/Lk is a promising candidate for the planned metalloading of preformed multi-tridentate polymers.
