Pouilly, S., Piller, V. and Piller, F.
[Metabolic Glycoengineering through the Mammalian GalNAc-salvage Pathway
>http://onlinelibrary.wiley.com/doi/10.1111/j.1742-4658.2011.08448.x/abstract;jsessionid=0F7FC54BD5CD490B913C2064E6B54D75.d01t04?systemMessage=Wiley+Online+Library+will+be+disrupted+4+Feb+from+10-12+GMT+for+monthly+maintenance]
FEBS Journal (2012) 279, 586-598
Abstract:
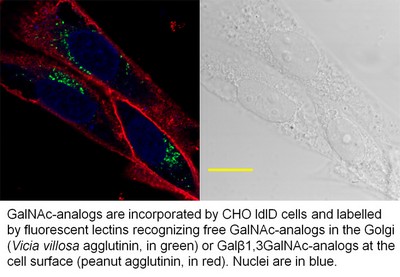
GalNAc is the initial sugar of mucin-type O-glycans, and is a component of several tumor antigens. The aim of this work was to determine whether synthetic GalNAc analogs could be taken up from the medium and incorporated into complex cellular O-glycans. The cell line employed was CHO ldlD, which can only use GalNAc and Gal present in the medium for the synthesis of its glycans. All GalNAc analogs with modified N-acyl groups (N-formyl, N-propionyl, N-glycolyl, N-azidoacetyl, N-bromoacetyl, and N-chloroacetyl) were incorporated into cellular O-glycans, although to different extents. The GalNAc analogs linked to Ser or Thr could be extended by the β3-galactosyltransferase glycoprotein-N-acetylgalactosamine 3β-galactosyl transferase 1 in vitro and in vivo and by α6-sialyltransferase α-N-acetylgalactosaminide-α-2,6-sialyltransferase 1. At the surface of CHO ldlD cells, all analogs were incorporated into sialylated O-glycan structures like those present on wild-type CHO cells, indicating that the GalNAc analogs do not change the overall structure of core-1 O-glycans. In addition, this study shows that the unnatural synthetic GalNAc analogs can be incorporated into human tumor cells, and that a tumor antigen modified by an analog can be readily detected by a specific antiserum. GalNAc analogs are therefore potential targets for tumor immunotherapy.