2022 is the year of the France-Portugal cooperation, organized by the French and Portuguese Ministries for Europe, Foreign Affairs and Culture.
This “France-Portugal 2022” season is an opportunity to highlight many events marking the cooperation between the two countries, including several in the field of Higher Education and Research.
In this context, the Ministry of Higher Education, Research and Innovation and the Academy of Sciences are pleased to propose a scientific prize which distinguishes existing cooperation between French and Portuguese teams, and to offer them an opportunity to deepen the cooperation in the years to come. The four prizes awarded in 2022 cover all scientific disciplines.
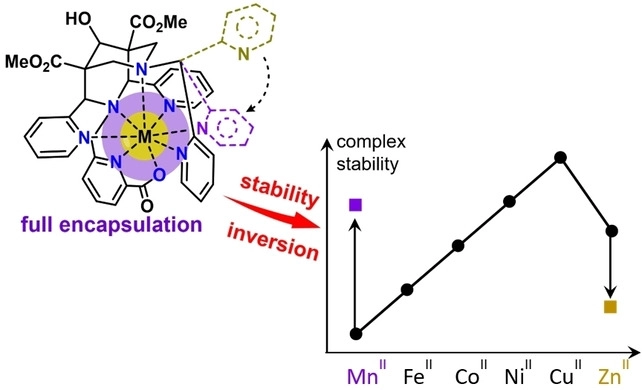
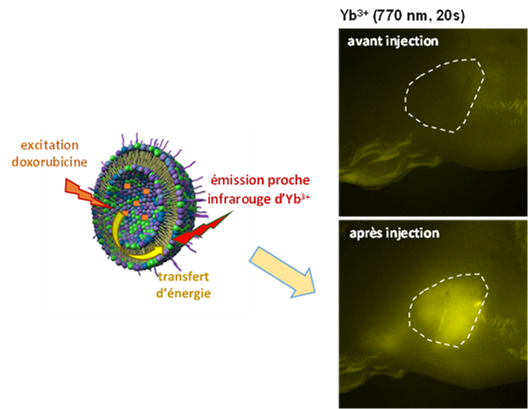
In this frame, the collaboration carried out for many years by Eva Jakab Toth with the University of Coimbra has been rewarded with the Mariano Gago Prize. This collaboration concerns the development of imaging agents based on metalloporphyrins for the detection of tissue redox states.
The official award ceremony helded at the Academy of Sciences on Tuesday, June 21, 2022.