LNPs are a leading class of mRNA delivery systems. LNPs are made of an ionizable lipid, a polyethyleneglycol (PEG)-lipid conjugate and helper lipids. The success of LNPs is due to proprietary ionizable lipids and appropriate helper lipids.
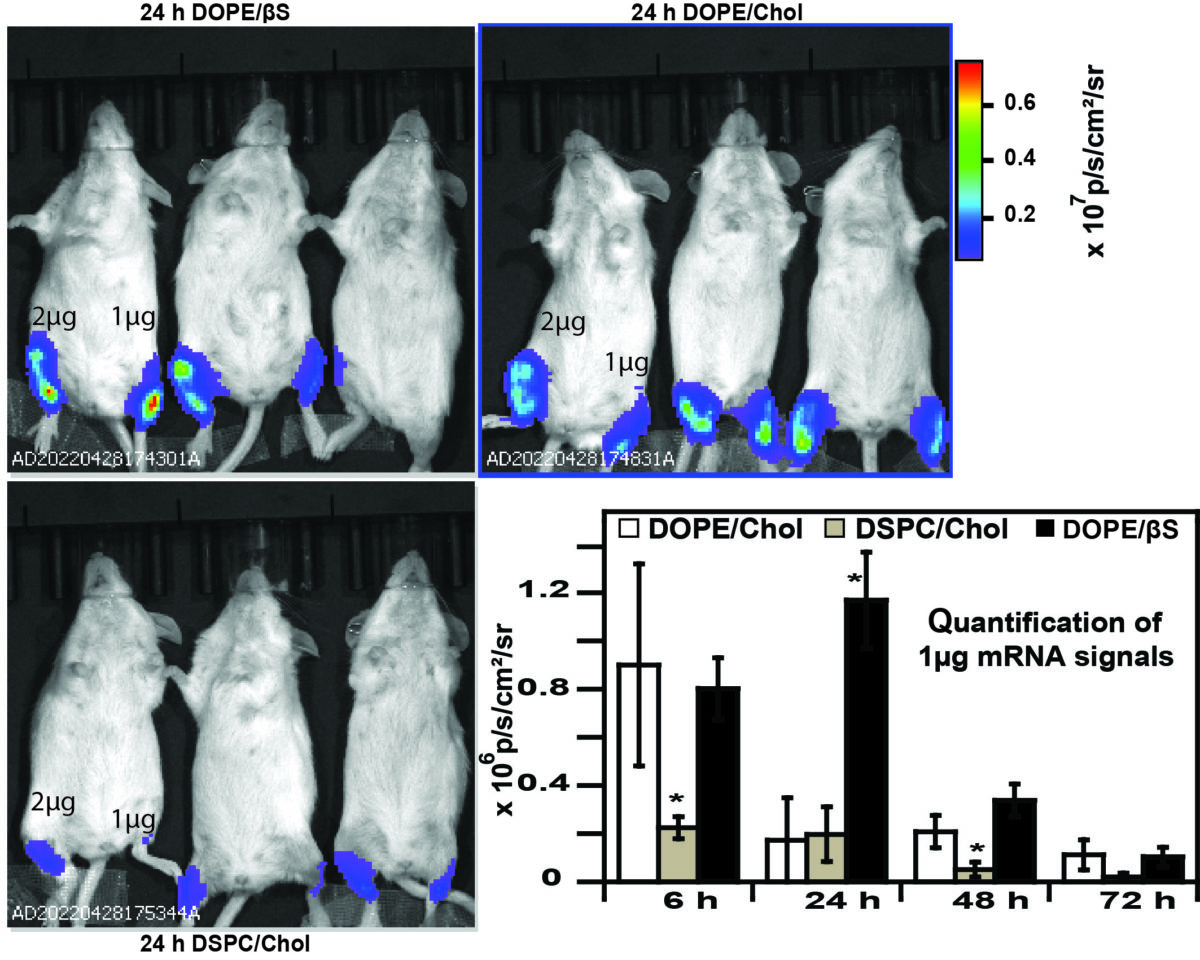
Using a benchmark lipid (D-Lin-MC3) researchers compared the ability of three helper lipids to transfect dendritic cells in cellulo and in vivo. Studies revealed that the choice of helper lipid does not influence the transfection efficiency of immortalized cells but, LNPs prepared with DOPE (dioleylphosphatidylethanolamine) and β-sitosterol were more efficient for mRNA transfection in murine dendritic cells than LNPs containing DSPC (distearoylphosphatidylcholine).
This higher potency of DOPE and β-sitosterol LNPs for mRNA expression was also evident in vivo but only at low mRNA doses.
References of the article published in Nanomaterials:
Ayoub Medjmedj, Albert Ngalle-Loth, Rudy Clemnçon, Josef Hamacek, Chantal Pichon and Federico Perche
In Cellulo and In Vivo Comparison of Cholesterol, Beta-Sitosterol and Dioleylphosphatidylethanolamine for Lipid Nanoparticle Formulation of mRNA
Nanomaterials 2022, 12(14), 2446; https://doi.org/10.3390/nano12142446