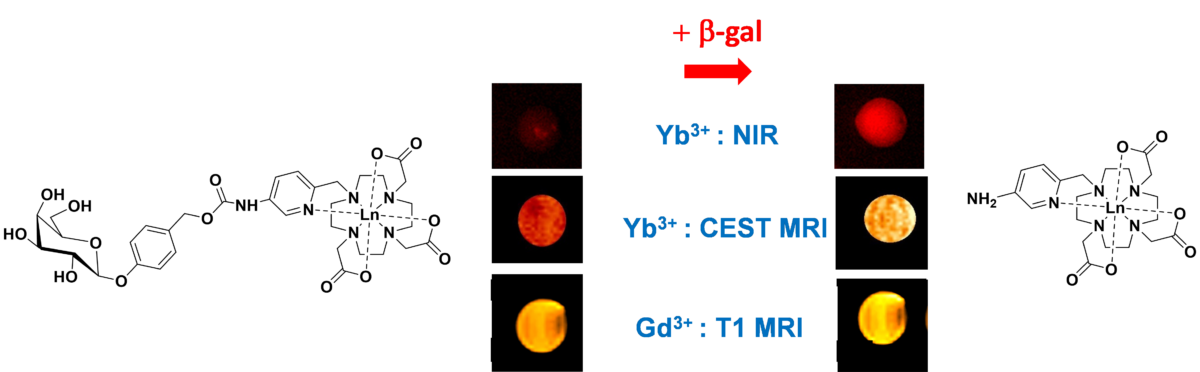
Scientists at the CNRS Centre de Biophysique Moléculaire (CBM) in Orléans and the Institut de Chimie des Substances Naturelles (CNRS/Université Paris-Saclay) have designed luminescent probes. They are based on complexes of lanthanides (Ln), a series of rare-earth metals whose trivalent Ln3+ ions are luminescent. The special feature of these probes is that the activity of certain enzymes can modify their luminescence in the near infrared, as well as the signal observed on MRI. These probes make it possible to track the catalytic activity of an enzyme with a single molecule using several complementary imaging techniques: MRI and near-infrared optics. Essential for unambiguous detection of a biological phenomenon, this dual imaging with a single molecule avoids biases due to the use of chemically different imaging agents for each imaging technique.
This study, published in the journal Angewandte Chemie International Edition, paves the way for new non-invasive diagnostic strategies.
This work has been reported on the Cnrs Chimie website
Article reference
Lanthanide-Based Probes for Imaging Detection of Enzyme Activities by NIR Luminescence, T1- and ParaCEST MRI
Rémy Jouclas, Sophie Laine, Svetlana V. Eliseeva, Jérémie Mandel, Frédéric Szeremeta, Pascal Retailleau, Jiefang He, Jean-Francois Gallard, Agnès Pallier, Célia S. Bonnet, Stéphane Petoud, Philippe Durand & Éva Tóth
Angew. Chem. Int. Ed. 2024
https://onlinelibrary.wiley.com/doi/10.1002/anie.202317728