Typologie d'actualités: Group NMR of Biomolecules
Antimicrobial peptides: an atypical double-domain avian defensin, specifically found in eggs, reveals multiple roles in protection of the embryo
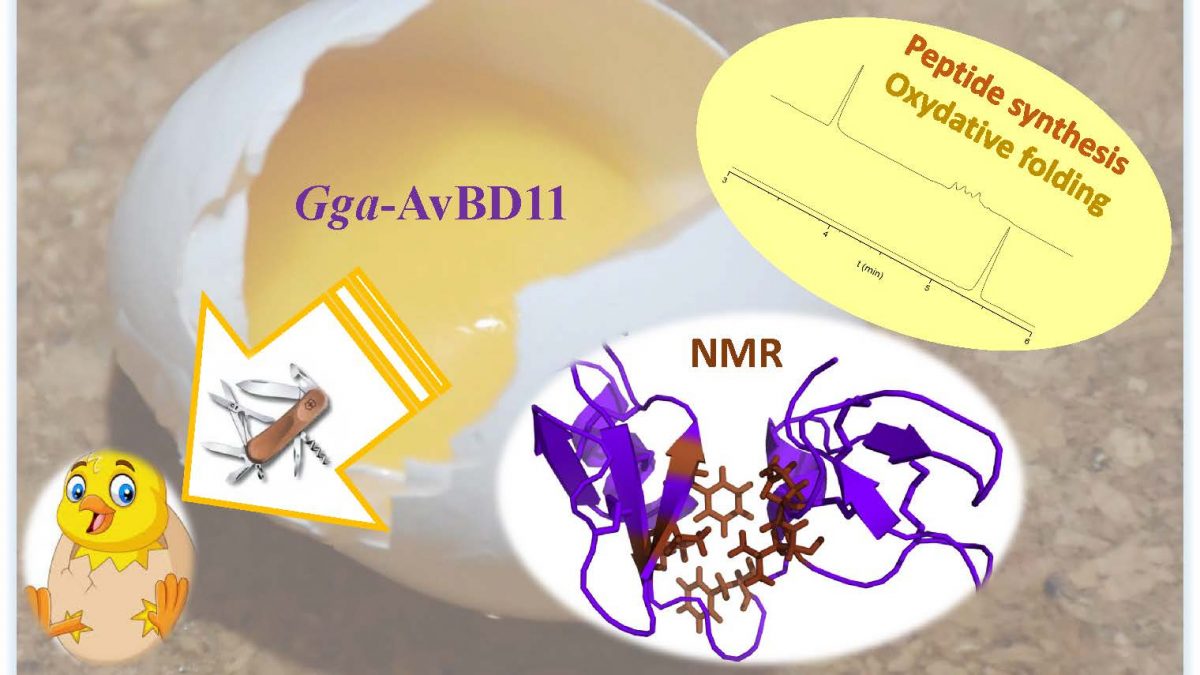
Gga-AvBD11, the avian β-defensin 11 of the common chicken Gallus gallus (Gga-AvBD11), is egg-specific, and represents the sole double-sized defensin (9.3 kDa) among the 14 AvBDs reported in the chicken species. The appearance of such a double-domain protein during evolution could be driven either by its increased biological potency compared to a single domain molecule, and/or by the necessity to acquire new functions carried only by the full-length protein. To assess the contribution of the two domains, we chemically synthesized them. We determined the 3D NMR structure each domain, and the structure of the compact full-length Gga-AvBD11, composed of two packed β-defensin domains. There is no reference for such a double-β-defensin in structural databanks. Thus, AvBD11 is the archetype of a new structural family, which we named avian-double-β-defensins (Av-DBD).
Its high sequence conservation among birds suggests its essential roles in the avian egg. In collaboration with several teams (Nouzilly and Tours, France), we showed that Gga-AvBD11 displays antimicrobial activities against Gram+ and Gram- bacterial pathogens, the avian protozoan Eimeria tenella and avian influenza virus (H1N1). It also shows cytotoxic and anti-invasive activities, suggesting that it may be involved in the (re-)modeling of embryonic tissues. Our results point to a critical importance of the cationic N-ter domain in mediating antibacterial, antiparasitic and anti-invasive activities, with the C-ter domain potentiating the two latter activities. Strikingly, antiviral activity in infected chicken cells requires the full-length protein.
The benefit for the avian species of possessing a double-sized defensin is a fascinating question. In order to better understand the structure-activity and phylogenetic relationships of AvBD11s family, we are currently studying other AvBD11 proteins (SAPhyR-11 project grant from the Région Centre Val de Loire).
This work was funded by the MUSE (Grant no. 2014-00094512) and SAPhyR-11 (Grant no. 2017-119983) project grants from the Région Centre-Val de Loire.
Structure, function, and evolution of Gga-AvBD11, the archetype of the structural avian-double-β-defensin family
Nicolas Guyot, Hervé Meudal, Sascha Trapp, Sophie Iochmann, Anne Silvestre, Guillaume Jousset, Valérie Labas, Pascale Reverdiau, Karine Loth, Virginie Hervé, Vincent Aucagne, Agnès F. Delmas, Sophie Rehault-Godbert, and Céline Landon
PNAS January 7, 2020 117 (1) 337-345; first published December 23, 2019 https://doi.org/10.1073/pnas.1912941117
Antimicrobial peptides: How peptide chemistry and NMR shed light on the antimicrobial activity of big defensins
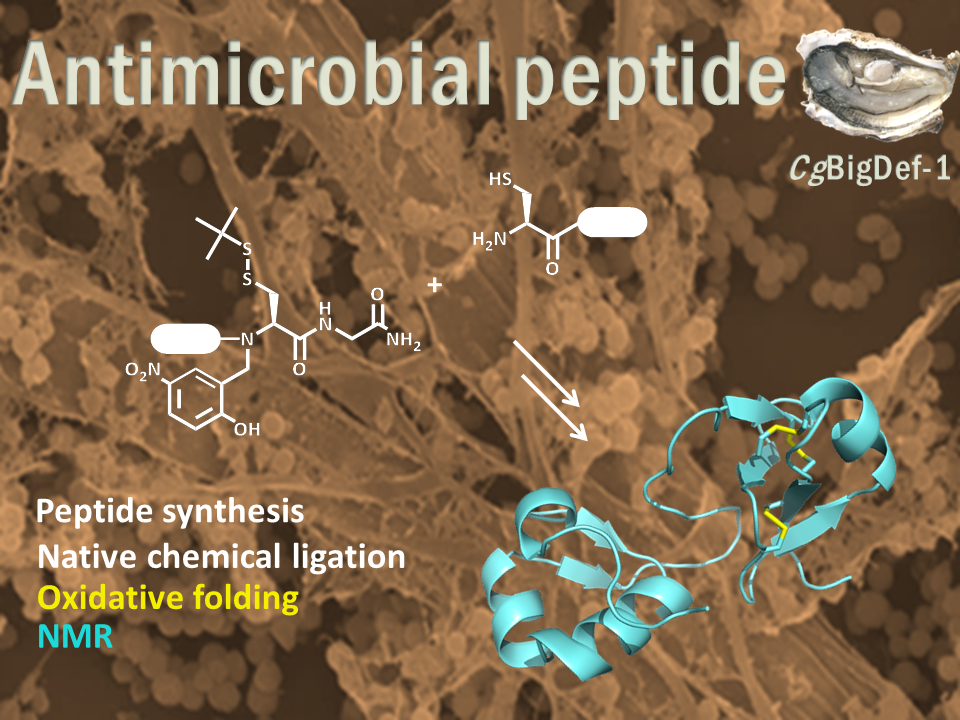
Big defensins, ancestors of β-defensins, are composed of a β-defensin-like C-terminal domain and a hydrophobic ancestral N-terminal domain.
This unique structure is found in a limited number of phylogenetically distant species, mostly living in marine environments. Using solid phase peptide chemistry and native chemical ligation, we produced the oyster Crassostrea gigas BigDef1 (Cg-BigDef1) and its separate domains and characterized their 3D structure by NMR. Cg-BigDef1 showed salt-stable and broad-range bactericidal activity, including against multidrug-resistant clinical isolates of S. aureus. We found that the ancestral N-terminal domain confers salt-stable antimicrobial activity to the β-defensin-like domain, which is otherwise inactive. Moreover, upon contact with bacteria, the N-terminal domain drives Cg-BigDef1 assembly into nanonets that entrap and kill bacteria. We speculate that the hydrophobic N-terminal domain of big defensins has been retained in marine phyla to confer salt-stable interactions with bacterial membranes in environments where electrostatic interactions are impaired.
Those remarkable properties open the way to future drug developments when physiological salt concentrations inhibit the antimicrobial activity of vertebrate β-defensins (ANR MOSAR-Def 2019-2023).
Many thanks to D. Destoumieux-Garzón for collaboration, to “Vaincre La Mucovidose“ and CNRS PEPS X-life” for funding.
October 25th, 2019: External seminar Dr Jérôme LEPRINCE –




