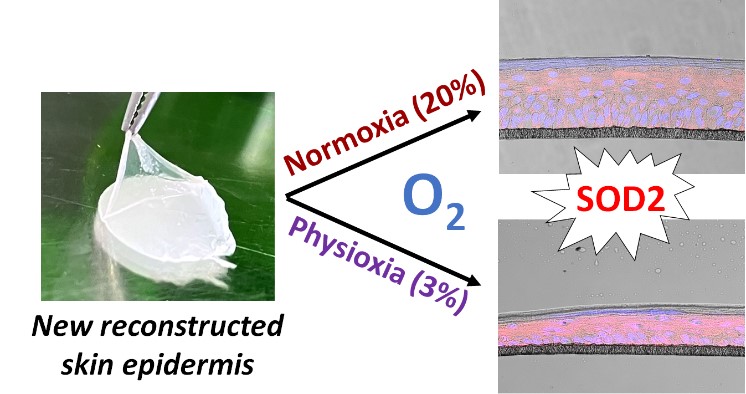
Skin in vitro 3D models, with varying degrees of complexity, are all developed under ambient air oxygen conditions, i.e. 18-20%, and are widely used to study the mechanisms governing skin functions or to screen numerous molecules for pharmaceutical or cosmetic purposes. However, in the skin, the physiological oxygen level is much lower, particularly in the basal layer of the epidermis where it falls to between 1 and 3%. In in vitro culture, skin cells are therefore in hyperoxia. Are these models representative of the physiological state of our skin?
To investigate this, researchers in the "Skin Biology and Microenvironment" team have developed new 2D and 3D in vitro models under the oxygen conditions of the skin's physiological microenvironment. They have shown that oxygen levels influence keratinocyte proliferation, leading to morphological differences in reconstructed epidermis. As oxygen levels are important in the production of free radicals, molecules that accelerate skin ageing, the researchers studied the antioxidant defences of the cells in these cultures. They showed that antioxidant activity was increased in physiological conditions, either by over-expression or over-activation of enzymes.
This work shows that oxygen levels control the antioxidant defences of skin cells, and that it is important to take this parameter into account in order to reproduce physiological conditions as closely as possible.
Improvement of Antioxidant Defences in Keratinocytes Grown in Physioxia: Comparison of 2D and 3D Models.
Chettouh-Hammas N, Fasani F, Boileau A, Gosset D, Busco G, Grillon C.
Oxid Med Cell Longev. 2023 Jun 17;2023:6829931. doi: https://doi.org/10.1155/2023/6829931