Cooper in a micronutrient essential to life, its lack induces neurological and blood disorders. It is extensively used in industry, in particular in the batteries of electric cars, but also as a fertilizer and fungicide. However, it is toxic at high concentrations, and is considered as a critical emerging pollutant. Copper detection in water constitutes a major societal and environmental issue.
Currently, copper concentrations are monitored by sophisticated analytical methods requiring time, expansive equipments and deep expertises. Moreover, these technics quantify total copper present in a sample and not copper interacting with living organisms.
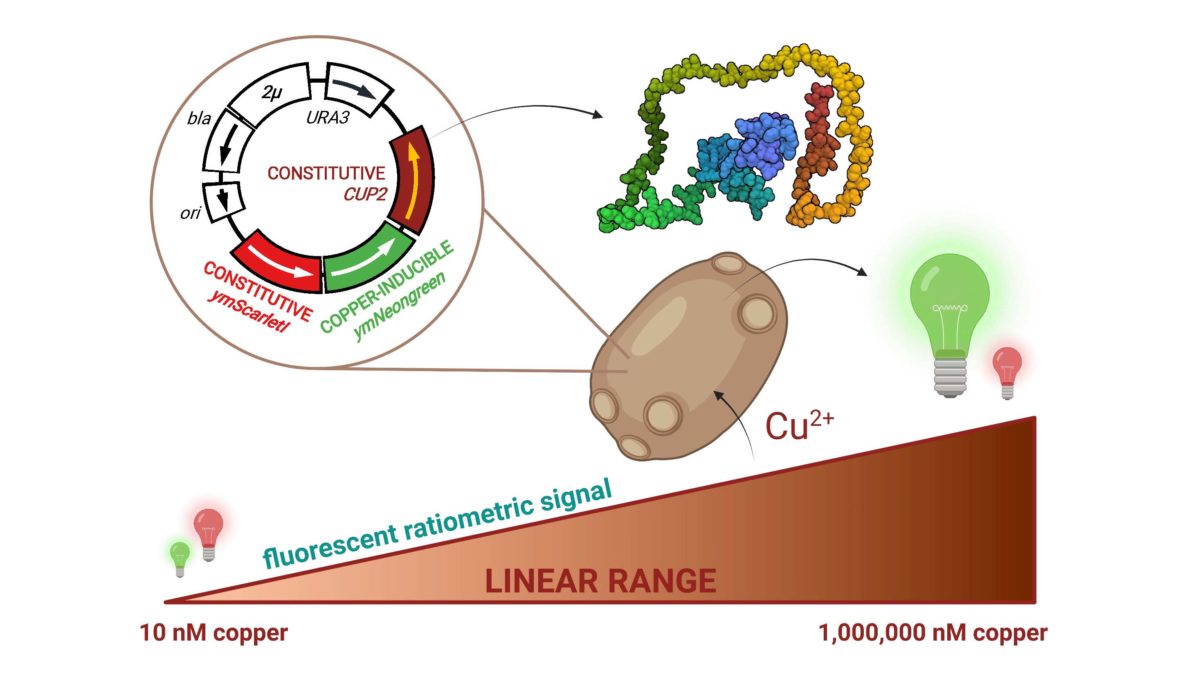
Researchers of CBM developed a new and atypical device able to detect copper in a very sensitive and easy way. Their biosensor is based on whole-cell eukaryotic living Saccharomyces cerevisiae, allowing detection of bioavailable copper. It is a ratiometric biosensor, measuring the ratio between (i) a constitutively expressed fluorescent protein and (ii) another fluorescent protein whose expression is directly correlated with copper concentrations as its expression is in under the control of CUP1 promoter, a well-known promoter in Saccharomyces.
By genetic engineering, researchers created many different variants to optimize the response of our biosensor. Their best biosensor is able to detect as low as 10 nM of copper in a linear range from 10-3 to 10-8 M, much better features compared to other currently reported whole cell copper biosensors. This biosensor was also validated on “real” samples: detected concentrations are totally in agreement with manufacturers’ values.
Reference of the article :
Bojan Zunar, Christine Mosrin, Hélène Bénédetti, Béatrice Vallée
Re-engineering of CUP1 promoter and Cup2/Ace1 transactivator to convert Saccharomyces cerevisiae into a whole-cell eukaryotic biosensor capable of detecting 10 nM of bioavailable copper
Biosensors and Bioelectronics 214 (2022) 114502
The article was reported by the CNRS Institute of Chemistry on its website and in its letter "En direct des labos".