Typologie d'actualités: Team Molecular, Structural and Chemical Biology
2024, April 18 – “La plongée, un sport sous pression”

A la MJC d'Olivet - Le Moulin de la Vapeur - 127 rue Marcel Belot
Jeudi 18 avril à 18h30 - Entrée gratuite mais sur réservation Par ici !
Avec Martine Beaufour, enseignante à l'université d'Orléans et chercheure au Centre de Biophysique Moléculaire, équipe "Spectrométrie de masse fonctionnelle des assemblages moléculaires"
Nos espaces aquatiques sont nombreux et parfois vastes : lacs, mers, océans... Quoi de mieux que la plongée sous-marine pour les explorer ? Faune, épaves, sites archéologiques, il y a tant de choses à voir !
Lors d’une plongée, tout plongeur s’expose à des variations de pression. Nous verrons pendant cette présentation comment la pression varie avec la profondeur en milieu aquatique, et en conséquence, comment la pression impacte la gestion d’une plongée sur de nombreux aspects.

Breast cancer: towards early diagnosis by imaging
In vivo imaging of metastatic breast cancer tumors at very early stages is about to become possible. A team of chemists and biologists from the Center for Molecular Biophysics (CNRS) has indeed developed a new magnetic resonance imaging (MRI) probe which has a selective affinity for an emerging biomarker of metastatic breast cancer: Netrin- 1. Find out more on the Cnrs Chimie website.
See more and Cnrs Chmie website.
Reference
Peptide-Conjugated MRI Probe Targeted to Netrin-1, a Novel Metastatic Breast Cancer Biomarker
Clémentine Moreau, Tea Lukačević, Agnès Pallier, Julien Sobilo, Samia Aci-Sèche, Norbert Garnier, Sandra Même, Éva Tóth & Sara Lacerda
Bioconjugate Chemistry 2024
https://doi.org/10.1021/acs.bioconjchem.3c00558

Details of the 4 thesis offers:
Thesis subject: "Suffering at work and consumption of psychoactive substances among territorial agents of the Communauté de commune des Terres du Val de Loire (TraPsyCOL)"
Supervisor: Raphaël Serreau, Neurobiology of receptors and therapeutic innovations team
View the offer
Thesis subject: "Molecular assembly of synthetic microRNAs in intracellular condensates of human glioblastoma cells"
Supervisors: Patrick Baril and Séverine Morisset-Lopez, Neurobiology of receptors and therapeutic innovations team
View the offer
Thesis subject: "Discovery and total chemical synthesis of D-proteins for molecular targeting of tumor glyco-epitopes"
Supervisors: Vincent Aucagne and Carlo Pifferi, Synthetic proteins and bioorthogonal chemistry team
View the offer
Thesis subject: "Anti-hOGG1 nanobodies to study the interactions between DNA repair and transcription regulation and to modulate the action of hOGG1 in anti-cancer strategies"
Supervisor: Bertrand Castaing, “Post-translational modifications of proteins and DNA repair: structure, functions and dynamism” team
View the offer
La ligue contre le Cancer (The League Against Cancer) supports research carried out at the CBM

Each year, the Grand Ouest Committee of the League Against Cancer actively participates in research by financing a certain number of scientific projects. At the start of 2024, he donated €84,000 to CBM researchers.
3 projects, at the cutting edge of cancer research, have been funded.
"New therapeutic approach in oncology: development of PROTACs targeting LIMK1 and LIMK2 kinases"
Leader: Béatrice Vallée co-leader of the “Cell signaling and neurofibromatosis” team
Partner: Karen Plé, ICOA, UMR7311, CNRS/University of Orléans
The protein kinases, LIMK1 and LIMK2, are involved in cell skeletal remo Their active role in the development of cancers has been shown both in the formation of tumors and in their dissemination and the growth of metastases. Targeting LIMK1 and LIMK2 to develop new anti-cancer therapies is therefore a very relevant strategy.Unfortunately, traditional small chemical molecules inhibiting the activity of LIMK1 and LIMK2 have not passed clinical trials.In our project, we therefore decided to develop a new class of molecules, PROTACs, which allow the destruction of their target directly in the cell. In our case, we target the destruction of LIMK1 and LIMK2 to annihilate their oncological activity. This PROTAC strategy is very innovative and growing. Building on very promising results that have been obtained on other therapeutic targets, we hope to demonstrate the proof of concept of this new approach targeting LIMK1 and LIMK2, and thus open the way to new therapeutic molecules.
This project received the support of the League against Cancer, the Committees of Loiret, Loir-et-Cher and Morbihan, for the sum of €32,000.
“Innovative prostate cancer diagnosis for personalized medicine approaches: intelligent multiplex mapping of SKCa channels using near-infrared emitting lanthanide-based metallacrowns”
Leader: Svetlana ELISEEVA, “Luminescent lanthanide compounds, spectroscopy and optical bioimaging” team
Each patient's tumors are different because each individual is different. It is therefore crucial to take this diversity of tumors into account to develop personalized approaches. Our research work aims to characterize cancerous tumors with the aim of providing more effective treatment to cure cancer.Several specific potassium channels play a major role in the progression of cancerous tumors and are sensitive to therapies. In this project, we are implementing an innovative near-infrared optical imaging approach which will make it possible to establish the precise identity map of these potassium channels for each patient in order to provide personalized treatment.
This project received the support of the League against Cancer of the Committees of Loiret, Loir-et-Cher and Sarthe, for the sum of €30,000.
“Cancer immunotherapy: evaluation of synthetic versus in-cell expressed bispecific di-affibodies”
Leader: Josef Hamacek, head of the “Molecular assemblies and complex systems” team
Partners: Federico Perche, Vincent Aucagne (CBM), Florence Velge-Roussel (NMNS Tours)
Several receptors on the surface of cancer cells may represent potential targets for antibodies with broad neutralization spectrum. The principle of bispecific synergistic antibodies is to bind via a binding site on the surface of the tumor cell and with the other site to a receptor on the surface of the immune effector cell (NK, T lymphocytes).In this context, antibodies can be replaced by affibodies (AfBs) presenting affinities and selectivities for their targets comparable to antibodies, but having smaller size.This project aims to develop bispecific di-AfBs as innovative agents for the diagnosis and immunotherapy of cancer.These complex molecules are made up of two AfBs linked by linkers and can thus target two epitopes. AfBs will bind specifically to the corresponding receptors overexpressed on the surface of cancer cells to block signaling pathways, and to promote the recruitment of effector cells.This concept offers new therapeutic perspectives and makes it possible to optimize interactions with the cell and provoke the immune reaction.
This project received support from the League Against Cancer of the Committees of Loiret, Sarthe and Côtes d’Armor, for the sum of €22,000.
Marie Curie Postdoctoral Fellowships
Rafael Aroso, from the University of Coimbra, Portugal, will join the “Metal Complexes and MRI” team. Its “PorphIRON” project aims to develop paramagnetic complexes based on transition metals, such as iron, for MRI applications. This work is part of the team's efforts to replace gadolinium, currently used in MRI, with more biocompatible and ecologically more sustainable metals.
Ross Ballantine, from Queen's University Belfast in Northern Ireland, will join the "Synthetic Proteins and Bioorthogonal Chemistry" team to work on the « ThioSHowcase » project. This project aims to explore arylthiol-based amino acids for the chemoselective functionalisation of peptides and of proteins, with applications in protein labeling and selective peptide cross-linking.
Congratulations to Lylia Azoug for her poster prize!

Lylia Azoug, PhDstudent in the “Synthetic protein and bioorthogonal chemistry” team, received the poster prize during the “Chemical Biology Symposium 2024” conference organized by the Société Chimique de France.
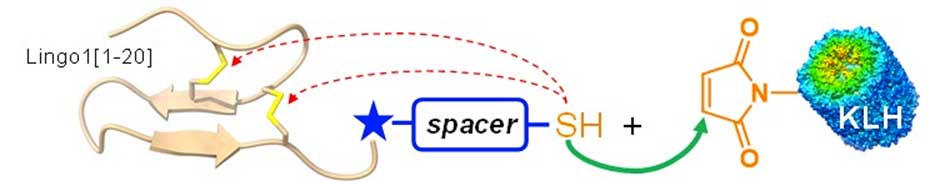
Poster abstract :
Generation of specific antibodies against peptides requires their covalent conjugation to large protein carriers, such as keyhole limpet hemocyanin (KLH), to override their inherently weak immunogenicity. For this, most approaches exploit the highly-efficient thiol-maleimide ligation, which enables for the attachment of multiple copies of peptides displaying a single cysteine thiol group, onto a variety of commercial maleimide-functionalized carriers. Concerning disulfide-containing peptides and proteins, introduction of a spare thiol moiety permits a follow-up thiol-maleimide reaction; nevertheless, disulfide bonds are highly susceptible to undergo intramolecular thiophilic attack by the thiolate required for the reaction, with consequent impairing of their native bridging framework. This “disulfide scrambling” would alter the peptide 3D structure, impairing specificity of elicited antibodies for the native target. In recent reports, thiol-functionalized spacers were introduced on disulfide-containing peptides to generate protein conjugates for immunization purposes, 1,2 nevertheless the occurrence of disulfide scrambling was not examined, therefore we sought to systematically evaluate the influence of different thiol-functionalized spacers. Using the N-terminal domain of the Lingo1 protein as a model peptide (20 amino acids, 2 disulfide bridges), we probed a small battery of thiol-containing spacers with variable length and rigidity. Interestingly, while flexible linkers, comparable with previously described ones,1,2 showed considerable disulfide scrambling upon 2 h under thiol-maleimide reaction conditions (pH 6.6), a short and rigid oligoproline-based linker3 abolished the disruption of the disulfide framework. The reported strategy provides a valuable resource for the bioconjugation of disulfide-containing peptides via standard thiol-maleimide chemistry while maintaining the structural integrity of the disulfide framework, thereby providing homogeneous tools for research and biomedical applications.
- Katayama, H.; Mita, M. A sulfanyl-PEG derivative of relaxin-like peptide utilizable for the conjugation with KLH and the antibody production. Bioorganic & ; Med. Chem. 2016, 24 (16), 3596–3602. DOI : 10.1016/j.bmc.2016.05.068
- Katayama, H.; Mizuno, R.; Mita, M. A novel approach for preparing disulfide-rich peptide-KLH conjugate applicable to the antibody production. Biosci. Biotechnol. Biochem. 2019, 83 (10), 1791–1799. DOI : 10.1080/09168451.2019.1618696
- Wilhelm, P.; Lewandowski, B.; Trapp, N.; Wennemers, H. A Crystal Structure of an Oligoproline PPII-Helix, at Last.J. Am. Chem. Soc. 2014, 136 (45), 15829–15832. DOI : 10.1021/ja507405j


